Press Release
Teleradiology Market Forecasted to Witness Noteworthy Growth by 2030 – Agfa-Gevaert, Siemens Healthineers AG
Pune, Maharashtra, India, 12th Feb 2024 – The latest report by Congruence Market Insights, titled ‘Global Teleradiology Market – Size, Trends, Share, Growth, Dynamics, Competition, and Opportunity Forecast, 2023 – 2030’, provides a thorough analysis of the global teleradiology market. The report meticulously examines both macro and micro trends, offering insights into the dynamic factors influencing the market. It encompasses a detailed exploration of qualitative and quantitative aspects, delivering a precise depiction of market size, growth rates, annual progression, prevailing trends, key drivers, promising opportunities, and potential challenges. Additionally, the report highlights the impact of crucial events such as technological advancements and regulatory frameworks on the teleradiology market landscape. This exhaustive examination equips businesses and stakeholders with invaluable intelligence for making informed decisions in the evolving teleradiology industry.
Request full report sample here: https://www.congruencemarketinsights.com/report/teleradiology-market?section=Request
What is the anticipated growth rate till 2030, along with the major drivers, restraints, and opportunities?
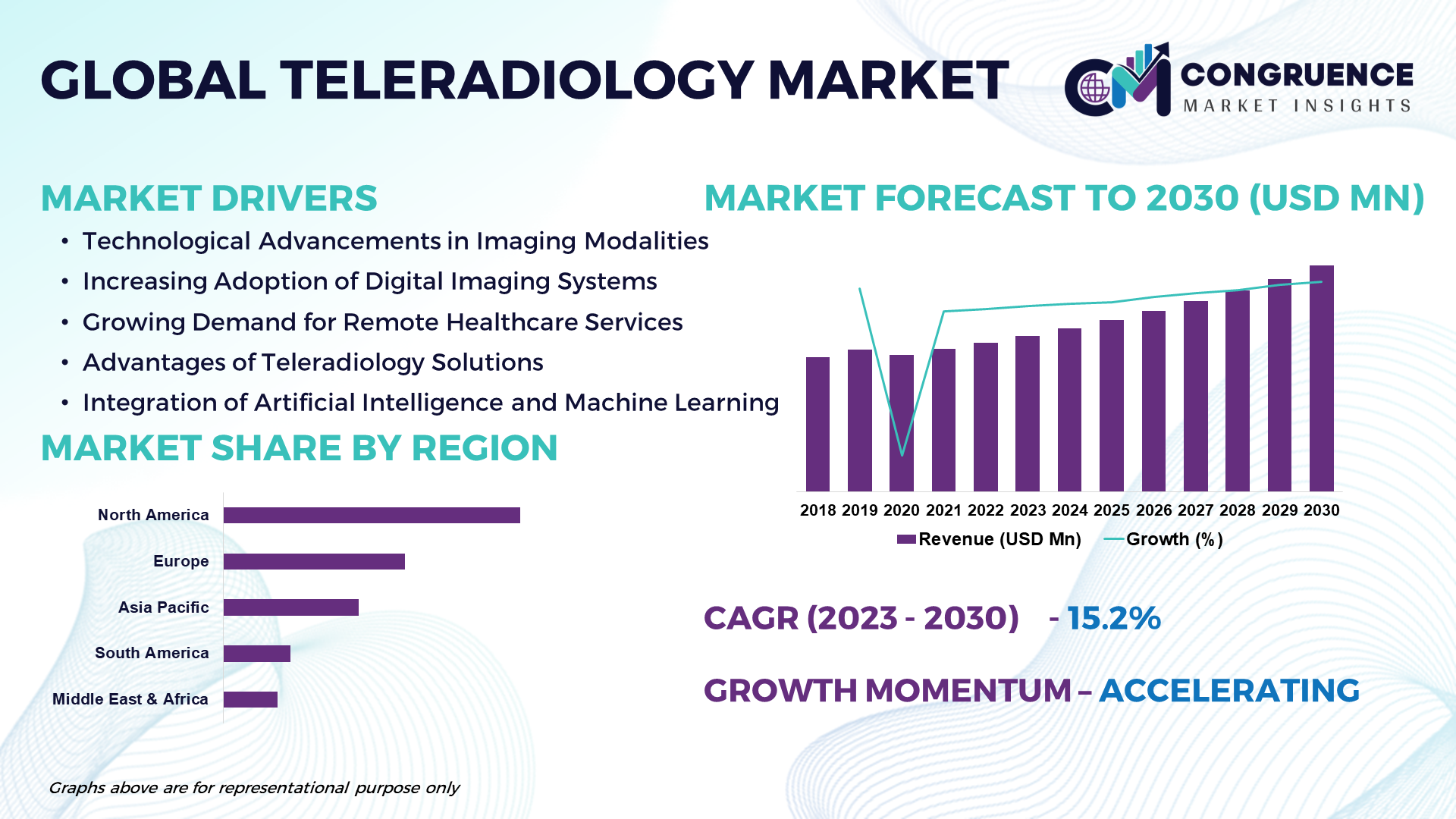
According to the in-depth market study, the global teleradiology market is anticipated to expand at a CAGR of 15.2% between 2023 and 2030. The teleradiology market is driven by the increasing demand for remote diagnostic services, advancements in imaging technology, and growing adoption of digital health solutions. The expansion of telemedicine infrastructure, coupled with the shortage of radiologists in certain regions, is expected to fuel market growth. Opportunities lie in the integration of artificial intelligence (AI) and machine learning algorithms for automated image analysis, expansion of teleradiology services in underserved areas, and strategic partnerships between healthcare providers and technology vendors to enhance patient care delivery. However, challenges such as data security concerns, regulatory complexities, and reimbursement issues may hinder market expansion.
How does AI impact the global Teleradiology market?
AI impacts the global teleradiology market by enhancing image interpretation, improving diagnostic accuracy, and optimizing workflow efficiency. AI-driven algorithms enable automated triage and prioritization of radiological studies based on clinical urgency, reducing turnaround times and improving patient care. Machine learning models facilitate the detection of abnormalities and subtle imaging findings, aiding radiologists in making accurate diagnoses. AI also supports the development of predictive analytics tools for identifying disease patterns and treatment response assessment. While AI integration enhances the capabilities of teleradiology services, challenges include the need for robust validation of AI algorithms, addressing ethical considerations in AI-guided diagnosis, and ensuring regulatory compliance in healthcare applications.
Buy Now @https://www.congruencemarketinsights.com/buy-now/328/1
Scope of the Report:
- Executive Summary
- Demand and Supply-side Trends
- Market Drivers, Restraints, Opportunities and Challenges
- Value Chain Analysis
- Porter’s Five Forces Analysis
- Industry SWOT Analysis
- COVID-19 Impact Assessment
- PESTLE Analysis
- Global Market Size and Forecast
- Regional Market Size and Forecast (Cross-country Analysis)
- Competition Landscape
- Company Profiles
Teleradiology Market Size and Forecast:
The report will comprehensively detail the teleradiology market size and forecast (2023-2030), presenting key metrics for strategic insights. We will analyze market revenue, quantifying total income from teleradiology hardware, software, and services, and provide volume insights into the usage of teleradiology solutions. The report will delineate market share, highlighting competitive landscapes. Year-on-Year growth analysis will track annual percentage changes, offering trend insights. Additionally, the Compound Annual Growth Rate (CAGR) will be presented, providing a smoothed growth rate for a more consistent assessment of the market’s expansion over the forecast period.
Which region holds the largest market share, and where does the major opportunity lie in the future?
The global teleradiology market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory frameworks, and technological adoption rates. North America currently holds the largest market share, attributed to the presence of established teleradiology service providers, favorable reimbursement policies, and the adoption of advanced imaging technologies. In the future, major opportunities are expected in Asia-Pacific, driven by increasing healthcare expenditure, growing demand for remote diagnostic services in rural areas, and initiatives promoting telehealth adoption. Other regions such as Europe, Latin America, and the Middle East & Africa also present growth opportunities, supported by the expansion of telemedicine networks and the rising prevalence of chronic diseases requiring imaging diagnostics.
Competition Landscape
The global teleradiology market is characterized by intense competition among key players striving for technological advancements and market leadership. Key competitors focus on product innovation, service quality, and strategic partnerships to gain a competitive edge. Companies such as Agfa-Gevaert Group, Siemens Healthineers AG, Fujifilm Holdings Corporation, and others dominate the market, offering a wide range of teleradiology hardware, software, and services catering to hospitals, diagnostic imaging centers, and ambulatory surgical centers. Market players are investing in research and development to introduce advanced imaging solutions, enhance interoperability, and improve patient outcomes. The competition landscape is dynamic, with a mix of established industry leaders and emerging players driving innovation and market growth in the teleradiology segment.
- Agfa-Gevaert Group
- Siemens Healthineers AG
- Fujifilm Holdings Corporation
- Koninklijke Philips N.V.
- GE Healthcare
- Carestream Health, Inc.
- Merge Healthcare Solutions, Inc.
- Teleradiology Solutions
- vRad (Virtual Radiologic)
- USARAD Holdings, Inc.
Comprehensive Market Segmentation:
- By Product Type (Hardware, Software, Services)
- By Modality (X-ray, CT, MRI, Ultrasound, Nuclear Imaging)
- By End-user (Hospitals, Diagnostic Imaging Centers, Ambulatory Surgical Centers)
Market Segmentation by Geography including:
- North America: U.S., Canada and Mexico
- Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe
- Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific
- South America: Brazil, Argentina, and Rest of Latin America
- Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa
Frequently Asked Questions (FAQs):
- What is the current market scenario?
- What was the historical demand scenario, and forecast outlook from 2023 to 2030?
- What are the key market dynamics influencing growth in the Global Teleradiology Market?
- Who are the prominent players in the Global Teleradiology Market?
- What is the consumer perspective in the Global Teleradiology Market?
- What are the key demand-side and supply-side trends in the Global Teleradiology Market?
- What are the largest and the fastest growing geographies?
- Which segment dominated and which segment is expected to grow fastest?
- What was the COVID-19 impact on the Global Teleradiology Market?
Explore in-depth industry research reports across various verticals from Congruence Market Insights @ https://www.congruencemarketinsights.com/reports/all-industries
Contact Us:
Ms. Shalaka Dubey
Senior Sales Manager
Congruence Market Insights
Palo Alto, CA 94301, United States
Phone: +1 650-646-2623
Email: sales@congruencemarketinsights.com
About Us:
Congruence Market Insights is a leading market research firm dedicated to providing in-depth analysis and strategic solutions for businesses across diverse industries. With a focus on delivering actionable insights, we offer comprehensive market intelligence, trend analysis, and forecasting to empower informed decision-making. We have built a reputation for delivering practical insights and genuine reports across diverse sectors covering an extensive array of both primary and niche sub-domains. Our expertise lies in uncovering market trends, consumer behavior, and competitive landscapes, enabling our clients to stay ahead in an ever-evolving business landscape.
Media Contact
Organization: Congruence Market Insights
Contact Person: Ms. Shalaka Dubey
Website: https://www.congruencemarketinsights.com/
Email: Send Email
Contact Number: +16506462623
Address: Palo Alto, CA 94301, United States
City: Pune
State: Maharashtra
Country: India
Release Id: 1202249660
The post Teleradiology Market Forecasted to Witness Noteworthy Growth by 2030 – Agfa-Gevaert, Siemens Healthineers AG appeared first on King NewsWire. It is provided by a third-party content provider. King Newswire makes no warranties or representations in connection with it.
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
China Medical System:First Ruxolitinib Cream’s Prescriptions for Vitiligo Issued in the Greater Bay Area
SHENZHEN, CHINA – China Medical System Holdings Limited (the “Group” or “CMS”) is pleased to announce that on 18 October, the first batch of prescriptions of ruxolitinib phosphate cream (the “ruxolitinib cream” or the “Product”) for qualified vitiligo patients were issued in the Greater Bay Area, at Zhongshan Chen Xinghai Hospital of Integrated Traditional Chinese and Western Medicine, Foshan Fosun Chancheng Hospital, and Dongguan Songshan Lake Tungwah Hospital. The Product’s new drug application (NDA) was approved by the Pharmaceutical Administration Bureau (ISAF) of Macau on 11 April 2024, and subsequently the Product was approved by the Guangdong Provincial Medical Products Administration on August 19 through the “Hong Kong and Macau Medicine and Equipment Connect” policy, which officially introduced ruxolitinib cream for the treatment of non-segmental vitiligo with facial involvement in adults and adolescents from 12 years of age, providing a novel treatment option for patients with relevant indication into designated medical institutions in the Mainland of Greater Bay Area.
In addition, on 24 September, the NDA for vitiligo indication of ruxolitinib cream has been accepted by the National Medical Products Administration of China (NMPA). In accordance with the relevant regulations of the drug real-world data application pilot program in the Hainan Boao Lecheng International Medical Tourism Pilot Zone (the “Pilot Zone”), CMS has conducted a real-world study on ruxolitinib cream in China. The results have shown positive efficacy, which is consistent with the key outcomes of global pivotal clinical studies. All secondary efficacy endpoints showed a trend of benefit consistent with the primary efficacy endpoint, and the treatment effect for vitiligo continued to improve with longer treatment duration. Meanwhile, through the safety monitoring data of the Pilot Zone, no new safety events have been identified. Adverse events mostly had severity levels of grade 1 or 2. No adverse event (AE) leading to discontinuation or withdrawal, and no serious adverse event (SAE) related to the study drug occurred.
If the Product is successfully approved for marketing in Mainland China, it will be the first prescription drug approved by NMPA for repigmentation in vitiligo, bringing this novel treatment hopes for Chinese vitiligo patients.
Furthermore, on 12 August 2023, the Product was approved by Hainan Medical Products Administration for Urgent Clinical Import, and officially became available to applicable patients in the Pilot Zone on August 18, for the topical treatment of non-segmental vitiligo in adults and adolescents aged 12 and above with facial involvement. Benefiting from the Early and Pilot Implementation Policy granted by the state to Hainan Free Trade Port and the Pilot Zone, patients with vitiligo in China can apply for the Product in Boao Super Hospital first and receive treatment from the expert team. As of 30 June 2024, more than 3,200 patients have been treated with ruxolitinib cream in Boao Super Hospital.
CMS has always been patient-oriented and innovation-driven based on clinical needs, continuously striving to improve drug accessibility. Benefited from the “Hong Kong and Macau Medicine and Equipment Connect” policy, ruxolitinib cream was approved for use in the Greater Bay Area and completed its first batch of prescriptions, shortening the time difference for Chinese vitiligo patients to use innovative drug and benefiting more domestic patients. Looking forward to the future, the Group will continuously strive to meet the unmet needs of Chinese patients, continuously explore novel drugs with international quality, and efficiently promote products’ clinical development and commercialization, so as to bring more quality pharmaceutical products through differentiated innovation-breakthrough, to safeguard the health and life-quality of patients.
About ruxolitinib cream
Ruxolitinib cream (Opzelura), a novel cream formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib, is approved by the U.S. Food & Drug Administration for the topical treatment of nonsegmental vitiligo in patients 12 years of age and older. As of now, it is the first and only treatment for repigmentation approved for use in the United States[1]. Ruxolitinib cream (Opzelura) is also approved in the U.S. for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) in non-immunocompromised patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable[2]. In Europe, ruxolitinib cream (Opzelura) is approved for the treatment of non-segmental vitiligo with facial involvement in adults and adolescents from 12 years of age[3].
On 2 December 2022, the Group through a subsidiary of the Company, a dermatology medical aesthetic company (“CMS Skinhealth”) entered into a Collaboration and License Agreement (the “License Agreement”) with Incyte for topical formulations of ruxolitinib for the treatment of autoimmune and inflammatory dermatology diseases. In accordance with the License Agreement, the Group through CMS Skinhealth received an exclusive license to develop, register and commercialize the Product in Mainland China, Hong Kong Special Administrative Region, Macau Special Administrative Region, Taiwan Region and eleven Southeast Asian countries (Indonesia, Philippines, Vietnam, Thailand, Myanmar, Malaysia, Cambodia, Laos, Singapore, Timor-Leste and Brunei Darussalam) (the “Territory”) and a non-exclusive license to manufacture the Product in the Territory. The License Agreement commenced on its effective date and has a royalty term of ten years from the date of the commercial sale of the Product in the Territory (the “Royalty Term”). Upon the expiration of the Royalty Term, the License Agreement may be renewed for a period of ten years thereafter (the “Initial Extended Royalty Term”) as per certain conditions defined in the License Agreement. Upon the expiration of the Initial Extended Royalty Term, the License Agreement may be extended for a period otherwise agreed by both sides as per certain conditions defined in the License Agreement.
Incyte has worldwide rights for the development and commercialization of the Product, marketed in the United States and Europe as Opzelura®. Opzelura and the Opzelura logo are registered trademarks of Incyte.
About vitiligo
Vitiligo is a chronic autoimmune disease characterized by depigmentation of the skin, which results from the loss of pigment-producing cells known as melanocytes. It is estimated that there are approximately 14 million vitiligo patients in China[4]. Non-segmental vitiligo patients account for approximately 85% of them. Topical corticosteroids (TCS) and calcineurin inhibitors (CI) are used off-label for non-segmental vitiligo, however, these therapies have clinical deficiencies with long-term adverse reactions of long-term treatment or limited efficacy[5、6].
About CMS
CMS is a platform company linking pharmaceutical innovation and commercialization with strong product lifecycle management capability, dedicated to providing competitive products and services to meet unmet medical needs.
CMS focuses on the global first-in-class (FIC) and best-in-class (BIC) innovative products, and efficiently promotes the clinical research, development and commercialization of innovative products, enabling the continuous transformation of scientific research into clinical practices to benefit patients.
CMS deeply engages in several specialty therapeutic fields, and has developed proven commercialization capabilities, extensive networks and expert resources, resulting in leading academic and market positions for its major marketed products. CMS continues to promote the in-depth development of its advantageous specialty fields and expand business boundaries. While strengthening the competitiveness of the cardio-cerebrovascular/gastroenterology business, CMS independently operates its dermatology and medical aesthetics business, and ophthalmology business, aiming to gain leading positions in specialty therapeutic fields, whilst enhancing the scale and efficiency. At the same time, CMS has expanded its business territory to the Southeast Asian market, striving to become a “bridgehead” for global pharmaceutical companies to enter the Southeast Asian market, further escorting the sustainable and healthy development of the Group.
Reference:
- Drug approval information can be found on the FDA official website, as follows: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-t…
- Drug approval information can be found on the Incyte official website, as follows: https://investor.incyte.com/news-releases/news-release-details/incyte-an…
- Drug approval information can be found on the EMA official website, as follows: https://www.ema.europa.eu/en/medicines/human/EPAR/opzelura
- Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74-84. doi:10.1016/S0140-6736(14)60763-7
- Consensus on the diagnosis and treatment of vitiligo (2021 version)
- Kubelis-López DE, Zapata-Salazar NA, Said-Fernández SL, Sánchez-Domínguez CN, Salinas-Santander MA, Martínez-Rodríguez HG, Vázquez-Martínez OT, Wollina U, Lotti T, Ocampo-Candiani J. Updates and new medical treatments for vitiligo (Review). Exp Ther Med. 2021 Aug;22(2):797. doi: 10.3892/etm.2021.10229. Epub 2021 May 25. PMID: 34093753; PMCID: PMC8170669.
CMS Disclaimer and Forward-Looking Statements
This press release is not intended to promote any products to you and is not for advertising purposes. This press release does not recommend any drugs, medical devices and/or indications. If you want to know more about the diagnosis and treatment of specific diseases, please follow the opinions or guidance of your doctor or other medical and health professionals. Any treatment-related decisions made by healthcare professionals should be based on the patient’s specific circumstances and in accordance with the drug package insert.
This press release which has been prepared by CMS does not constitute any offer or invitation to purchase or subscribe for any securities, and shall not form the basis for or be relied on in connection with any contract or binding commitment whatsoever. This press release has been prepared by CMS based on information and data which it considers reliable, but CMS makes no representation or warranty, express or implied, whatsoever, and no reliance shall be placed on, the truth, accuracy, completeness, fairness and reasonableness of the contents of this press release. Certain matters discussed in this press release may contain statements regarding the Group’s market opportunity and business prospects that are individually and collectively forward-looking statements. Such forward-looking statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties and assumptions that are difficult to predict. Any forward-looking statements and projections made by third parties included in this press release are not adopted by the Group and the Company is not responsible for such third-party statements and projections.
Media Contact
Brand: China Medical System Holdings Ltd.
Contact: CMS Investor Relations
Email: ir@cms.net.cn
Website: https://web.cms.net.cn/en/home/
Source: China Medical System Holdings Ltd.
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Unleash Your Creative Potential with CreateAI: The Future of AI-Enhanced Content Creation
The digital landscape is evolving at an unprecedented pace, and at the forefront of this transformation is CreateAI—an innovative platform that empowers creators with AI-enhanced tools to unlock their true potential. Whether you’re an aspiring influencer, a meme enthusiast, a business visionary, or a budding game developer, CreateAI offers the resources you need to craft captivating content that inspires and engages. With AI simplifying complex creative processes, the platform enables creators of all levels to effortlessly transform their ideas into reality.
A Platform for Every Creator
At its core, CreateAI is designed to democratize content creation by offering powerful, AI-driven tools that simplify the production of visuals, videos, music, and more. No matter your background or technical expertise, CreateAI equips you with the tools to innovate and produce high-quality content with ease.
The platform’s journey begins with Phase 1: Become an Influencer, where creators can master the art of content creation to engage their audience. With AI-enhanced tools, influencers can elevate their online presence, craft visually appealing posts, and produce captivating content that resonates with their followers. AI’s intuitive capabilities streamline the creative process, allowing you to focus on building authentic connections with your audience.
In Phase 2: Become a Meme Developer, CreateAI introduces an entirely new way to participate in internet culture. Memes have become a dominant form of communication in the digital age, and CreateAI makes meme creation fun and easy. By transforming text into shareable and humorous images, CreateAI’s tools empower users to create viral content effortlessly. Whether you’re aiming to grow your social media presence or simply express creativity, CreateAI brings your ideas to life.
Phase 3: Become a Businessman is for entrepreneurs and business leaders looking to harness the power of AI to elevate their strategies and content. CreateAI enables businesses to develop impactful campaigns, streamline branding efforts, and craft innovative ideas that drive growth. With AI-enhanced tools at your fingertips, you can focus on expanding your business while AI handles the heavy lifting of content creation.
In Phase 4: Become a Game Developer, CreateAI goes beyond content creation to offer an immersive game development experience. The platform’s AI tools allow users to create engaging games with stunning visuals and dynamic elements, all from text-based commands. Whether you’re a seasoned developer or someone new to game design, CreateAI simplifies the complex world of game development, making your vision a reality.
Powerful AI Tools for Every Creative Need
At the heart of CreateAI is a suite of AI-enhanced tools that empower creators to innovate across various mediums:
– /Create Avatar: Transform simple text into lifelike avatars, customized and animated to your specifications. Bring characters to life effortlessly with AI.
– /Create Music: Compose unique music tracks from text, enabling you to create original compositions without needing extensive musical knowledge.
– /Create Image: Generate stunning visuals from text, allowing you to bring your artistic ideas to life with ease.
– /Create Video: Produce engaging, high-quality videos from text-based inputs. Animate and edit seamlessly using AI assistance.
– /Create Meme: Design viral memes effortlessly with AI-driven tools, transforming text into shareable humor that captivates instantly.
– /Create Token Launch: Launch your own cryptocurrency tokens with AI precision, simplifying the process of entering the blockchain world.
– /Create Web: Build visually appealing, functional websites with the help of AI, streamlining the design and development process.
– /Create Game: Develop immersive games by leveraging AI to enhance game design, making the complex process of game development easy and intuitive.
A Strong Ecosystem Backed by Robust Tokenomics
CreateAI’s ecosystem is powered by its native token, $CREATE, which fuels the platform’s operations and growth. With a total supply of 100 million tokens on the Ethereum blockchain, the tokenomics of CreateAI ensures a balanced approach to liquidity, marketing, community incentives, and more. The ecosystem is designed to reward creators and foster a collaborative environment where both the platform and its users thrive.
Join the Future of Creativity
CreateAI is not just a tool—it’s a movement. By joining the CreateAI community, you become part of a vibrant network of creators, developers, and innovators. Whether you’re looking to elevate your content creation, develop new business strategies, or build immersive games, CreateAI provides the tools you need to succeed in today’s rapidly evolving digital landscape.
Start your journey today with CreateAI and transform your creative ideas into reality with AI-enhanced precision. Visit [CreateAI](https://www.createai.io) to learn more and join a new era of creativity.
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
Press Release
Launch Your Blockchain Project with Ease: Introducing CoinCreate
In today’s fast-paced digital economy, blockchain technology continues to revolutionize industries and open new opportunities for businesses and developers alike. However, the complexity of blockchain development has often acted as a barrier to entry, requiring specialized knowledge and technical expertise. CoinCreate, a cutting-edge platform, aims to change that by offering an all-in-one no-code solution that simplifies the process of launching blockchain projects. Whether you’re deploying tokens, creating staking contracts, launching NFT collections, or implementing governance systems, CoinCreate provides a seamless experience that empowers users to launch their blockchain projects with ease and speed.
One Platform, Multiple Solutions
CoinCreate offers a complete suite of tools designed to meet the needs of various blockchain projects, all accessible from a single platform. Gone are the days of relying on fragmented services for different aspects of your project—CoinCreate consolidates everything in one place. From deploying tokens to setting up governance structures, staking contracts, vesting contracts, and beyond, CoinCreate gives you the power to manage your entire project on a user-friendly platform.
Reach a Global Audience
The blockchain ecosystem is vast, with over 317 million blockchain users worldwide. CoinCreate enables you to tap into this massive audience by offering cross-chain compatibility with leading blockchains such as Ethereum, Arbitrum, Optimism, Binance Smart Chain (BNB), Avalanche, Polygon, and others. This means you can optimize for performance and cost on your preferred networks, while also reaching a broader and more diverse user base.
Cost-Effective Development Without the Complexity
One of the standout features of CoinCreate is its ability to eliminate the need for hiring specialized blockchain developers. Traditional blockchain development can be both costly and time-consuming, requiring expertise in smart contracts, tokenomics, and security protocols. CoinCreate removes this burden by providing an easy-to-use platform that allows software developers, teams, and businesses to deploy blockchain solutions without the steep costs associated with hiring niche talent. This allows you to focus on innovation, strategy, and community building while cutting down on development costs.
Intuitive Interface Designed for Everyone
CoinCreate has been thoughtfully designed to cater to users of all skill levels. Whether you’re an experienced blockchain developer or someone with minimal experience, CoinCreate’s simple interface ensures that you can deploy contracts effortlessly. The platform features an intuitive dashboard that provides a comprehensive view of your project, allowing you to manage your ecosystem efficiently. Detailed guides and tutorials also help users navigate the platform and deploy projects confidently.
Token Deployment in Minutes
Token deployment is one of the core features of CoinCreate, allowing users to create standard tokens, reflection tokens, and ERC404 tokens with just a few clicks. Customizing tokenomics has never been easier, and you can launch your token in minutes. This is especially valuable for businesses and projects that need to quickly launch tokens to engage their community, incentivize participation, or drive project development.
Engage Your Community with Staking and Governance
Staking contracts are another feature that makes CoinCreate stand out. With CoinCreate, you can set up staking contracts to incentivize token holding, ensuring long-term engagement from your community. Additionally, CoinCreate’s governance contract feature (coming soon) will enable you to build decentralized governance structures, empowering your community to participate in decision-making through transparent on-chain voting.
Future-Proof Features and Security
CoinCreate isn’t just about launching projects quickly—it’s about doing so securely and reliably. All contracts created through the platform are securely stored on the blockchain of your choosing. CoinCreate serves purely as an interface to the blockchain and does not hold or access any contracts. This ensures that your data remains in your control.
Furthermore, CoinCreate has integrated OpenZeppelin’s secure, audited smart contracts to provide an additional layer of protection. OpenZeppelin is a trusted name in blockchain security, offering modular contracts that are designed to protect decentralized applications (dApps) from vulnerabilities. This ensures that your contracts are built with best-in-class security from day one.
Who Can Benefit from CoinCreate?
CoinCreate caters to a wide range of users—from software developers and blockchain enthusiasts to Web3 projects, Web2 businesses looking to transition into blockchain, and even NFT artists and content creators. Whether you’re building complex decentralized applications, tokenizing assets, or simply experimenting with blockchain, CoinCreate provides the tools you need to succeed.
Ready to Build on the Blockchain?
CoinCreate is revolutionizing blockchain development by making it accessible, cost-effective, and simple for everyone. Whether you’re launching your first token, setting up staking contracts, or creating an entire decentralized ecosystem, CoinCreate is your all-in-one solution. Join the growing number of innovators already using CoinCreate to deploy their projects and unlock the potential of blockchain technology.
Important Links:
Website: https://coincreate.io/
X: https://x.com/CoincreateTeam
Telegram: https://t.me/CoinCreateio
About Author
Disclaimer: The views, suggestions, and opinions expressed here are the sole responsibility of the experts. No Digi Observer journalist was involved in the writing and production of this article.
-
Press Release1 week ago
Freedom FX Broker: Celebrating Three Consecutive Years as the Best Forex Broker
-
Press Release1 week ago
Empowering Companies to Cross Capital Market Boundaries: Carat W Holding’s Expertise in Listing and Fundraising in Taiwan, the U.S. and the U.K.
-
Press Release1 week ago
NEXT15 Innovative Advertising Work Platform, Creating a Win-Win Model for Users and Brands
-
Press Release1 week ago
Solana and Ethereum-Based Device Authentication and Authorization Consensus Algorithm Developed by Dono Vault
-
Press Release2 days ago
Indie Fest Carolina to Feature Dancehall/Reggae Sensation Remedy Live in Charlotte
-
Press Release2 days ago
How DLDCUSDT propels the EV industry forward
-
Press Release1 day ago
Growork: The groundbreaking SaaS platform set to revolutionise business connections in the UAE
-
Press Release2 days ago
SafetyXpress Launches Innovative U-Shaped Bollards for Maximum Protection in Commercial and Industrial Spaces



